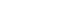Pioneering Additive Design
Lindsay Luminoso
Additive Manufacturing Medical 3D printing CT scanning RenishawRenishaw empowers medical device designers with specialized metal additive manufacturing and design software.

The additively manufactured guides used for facial reconstruction.
One of the biggest trends in medical device manufacturing is bespoke design. Gone are the days of one-size-fits-all devices, traded in for patient-specific implants. Advancements in new manufacturing processes and CAD software has enable the medical sector to move towards comprehensive care packages for patients. One-offs are easily produced through additive manufacturing, designed in the latest software developed with the best of both clinical and mechanical tools and next-gen technology.
Renishaw, a global engineering and scientific technology company with expertise in precision measurement and healthcare, has worked to bring design engineering to the medical sector for the best possible patient care. The company has a longstanding history with medical device manufacturing and has focused on dental restorations, neurological drug delivery systems and more recently maxillofacial implants.
The company has also focused its attention on developing additive manufacturing systems. On November 1, 2016, Renishaw opened its first North American Additive Manufacturing Solution Centre in Kitchener, ON, demonstrating its latest product, the RenAM 500M laser powder bed fusion additive manufacturing system. The Canadian team believes that medical device design and patient care will benefit greatly from the processing capability.
For Renishaw, additive manufacturing opens up many possibilities including partnering with industry professionals to design patient specific implants (PSI) with unique features that leverage the skills and specialities of both medical professionals and design engineers.
Skull Reconstruction
Because additive manufacturing is a recent phenomenon in the medical sector, Renishaw recognized the importance of partnering with leading experts in both the clinical and design engineering field to bridge the gap that previously existed with traditional processes.
In a recent collaboration, the company worked with Professor Adrian Sugar, a consultant in Cleft and Maxillofacial Surgery at Morriston Hospital in Swansea, UK, to design and develop surgical guides for a patient, Stephen Power, who suffered from multiple skull fractures.
Power underwent emergency surgery to reconstruct his face, but required additional surgeries to restore its symmetry. Renishaw was responsible for producing custom guides made from cobalt chrome alloy on its AM250 metal 3D printing machine. Using CT scans, the design team was able create patient specific guides to help surgeons place the implant with a near perfect fit and reconstruct Stephen Power’s face.
This first-of-its-kind partnership opened up new opportunities for collaboration between the medical and design engineering field. Traditional surgical boundaries were extremely limiting; additive manufacturing enabled Stephen Power to get the best possible care and ushered in a new era of medical device manufacturing, one that is centered on the patient.
A Canadian Perspective
Over the years, Renishaw has partnered with many UK-based companies to develop next-gen technologies for medical device manufacturing. However, with the opening of its new North American Additive Manufacturing Solution Centre, the company is exploring opportunities within the Canadian medical device market. One way is through collaboration with Dr. David Holdsworth, the Dr. Sandy Kirkley Chair of Musculoskeletal Research and scientist in the Imaging group of the Robarts Research Institute at Western University.
Additive manufacturing enables designers to include unique structures in medical device implants like lattice for bone adhesion.
This collaboration has proven that additive manufacturing opens design possibilities to develop structures that couldn’t be achieved with traditional manufacturing processes. One example of this is through the development of next-generation “smart” hip implants.
In conjunction with Western University, the team recently designed and 3D printed a device instrumented with electronic sensors and wireless telemetry in a porous implant. This hip joint prototype allows for sensors to monitor health-related data including strain, temperature, movement and the like.
“You can imagine in the IoT environment, the ability to use this data in healthcare opens up a lot of options, especially the type of data that wouldn’t be available to clinicians currently,” explains Matt Parkes, senior medical development engineer at Renishaw. “Whether that’s patient compliance, how quickly they get back on their feet when they are told to rest, or if there are micro-movements that are conducive to implant loosening; these are a good indicator of implant success/failure.”
Additive manufacturing enables the ability to adjust and adapt the device to meet patient needs. This process also allows for unique features to be incorporated into the design that are unheard of in traditional manufacturing. The hip implants produced through the partnership were modified to including an open-porous lattice structure, comprised of 1.5mm unit cells and 0.5mm struts. Parkes explains that this type of structure enhances bone ingrowth and device fixation.
“In healthcare, there’s lots of potential for novel ideas and concepts,” says Parkes. “3D printing has really opened up a whole host of new possibilities that weren’t there with subtractive techniques, whether that is lattice structures or a patient-specific result or the ability to quickly iterate a design and test a concept.”
What is more, designing patient specific implants is much easier with additive manufacturing. The cost of producing one-offs is significantly reduced without the added expense of tooling. Multiple iterations can be produced quickly and effectively to fit patient requirements.
A New Design Tool
One of the challenges with additively manufactured medical devices is in the design process. When it comes to traditional manufacturing, designers generally know about all of the manufacturing techniques of machining; however, in this case, the design engineer needs to also know about the clinical requirements.
“Those two sets of experiences aren’t typically naturally occurring,” explains Parkes. “There aren’t many 3D printing experts who happen to also be surgeons.”
He says that either design tools need to inform an engineer or manufacturer of clinical requirements or they need to enable a clinician to design implants that conform to manufacturing needs. Unfortunately, he says, there weren’t any suitable tools for this task. Of those that were capable of the organic shapes needed for implants, Parkes says they were extremely complex and had a steep learning curve.
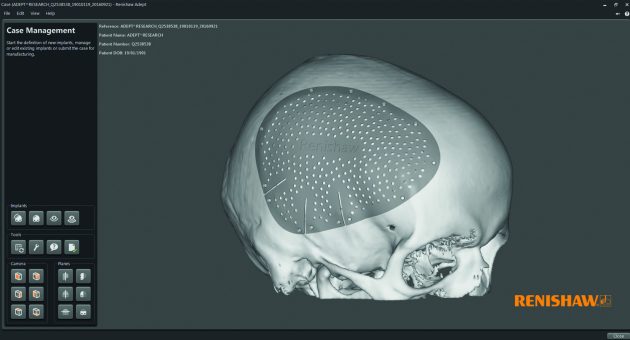
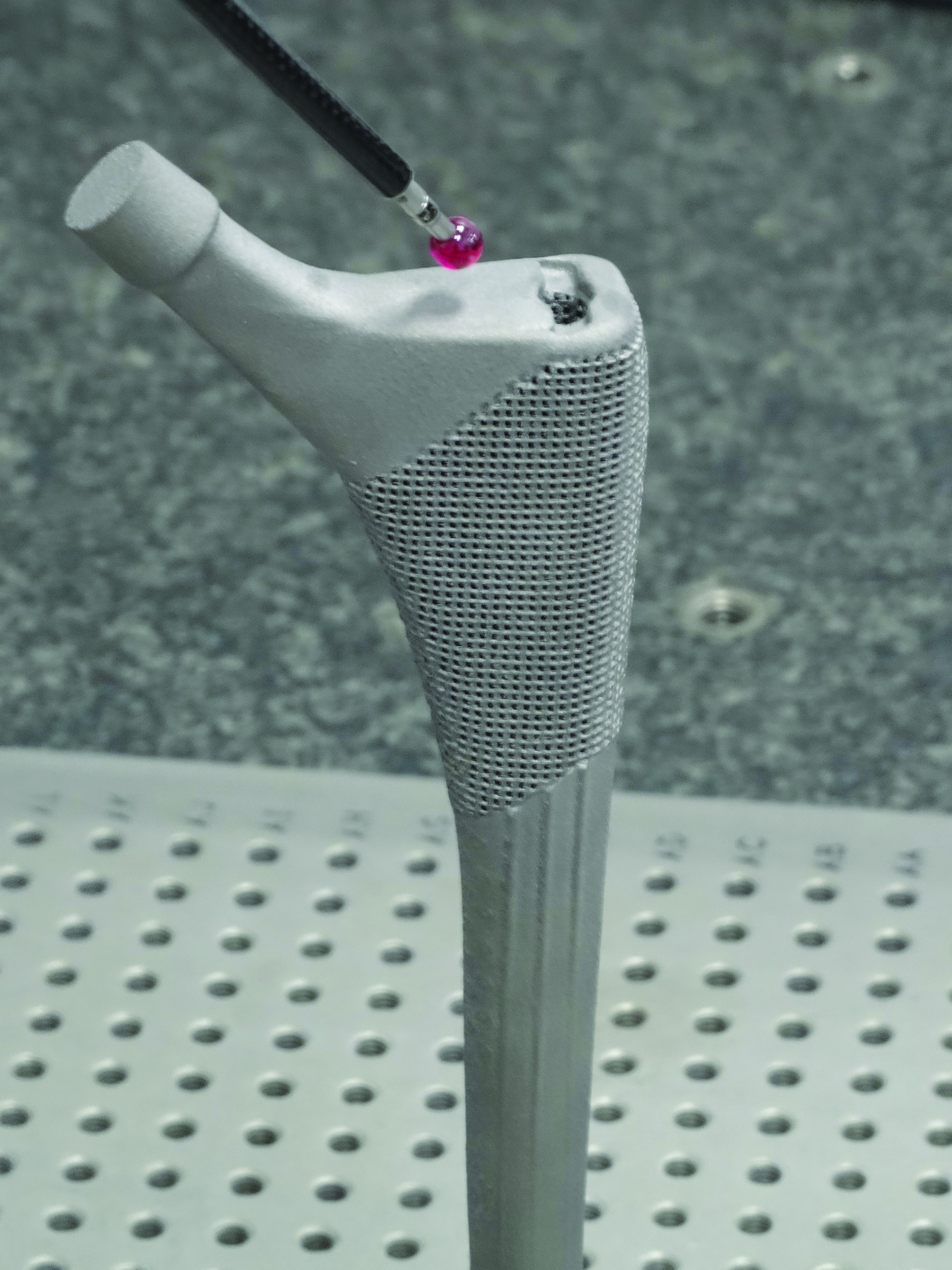
This new ADEPT software brings together both a clinical and engineering perspective to design the best possible medical devices.
“For example, a cranial plate could take upwards of a day to design with some of these tools,” he says. “Quite often, they aren’t built around an engineering or clinical approach. Some solely utilise freeform design tools, a design-whatever-you-want method; this lack of structured process isn’t particularly helpful for either approach.”
Renishaw is working to solve this problem with its ADEPT software, which was developed in-house by the Medical and Dental Products software team in conjunction with user experience experts PDR (Cardiff, UK) and clinical partner Abertawe Bro Morgannwg University Health Board (Swansea, UK).
“Our collaboration with end users has enabled us to develop a tool uniquely tailored to the clinical situation,” Parkes adds. The software is able to simplify the craniomaxillofacial (CMF) patient specific implant (PSI) design workflow through tools that easily achieve complex anatomical design.
Another challenge the team addressed was file format conversion. For the most part, when it comes to patient specific medical devices, designers start with CT scans and voxel-based data. Parkes explains that converting this data to an engineering surface can cause lots of issues. Working with surface features that are hard to identify on a CT scan and then accurately recreating them in a form that can then be designed or worked with is typically time consuming and difficult. However, the ADEPT software is able to dramatically streamline this process, allowing the user to quickly go from CT data through to design.
“Existing tools for PSI design are convoluted – requiring months of expertise to fully utilize, hours to complete simple cases and poor focus on the clinician’s requirements,” says Parkes. “By simplifying this process and offering intuitive tools, ADEPT will improve patient outcomes through reduction and removal of barriers to additive manufactured craniomaxillofacial patient specific treatment.”
The ADEPT software is currently in closed beta testing, but Renishaw says they have had positive feedback from clinicians and technicians in the UK and Europe. The software will be publicly released in early 2017.
“Ultimately we’d like to see PSIs used in the majority, not minority, of CMF cases, allowing patients and surgeons to exploit the benefits of custom fitting devices,” says Parkes. “ADEPT is a tool to help turn this into a reality.”
With the use of this software, the design of medical devices is becoming easier and with additive manufacturing, the devices are becoming highly customizable to meet patient needs. More medical professionals are recommending and opting for PSIs and Renishaw is helping advance this trend. Because the company also manufactures the additive manufacturing systems, they are in a position to assist from both the machine side and the design process.
“It’s a pretty unique situation,” Parkes says, “There are lots of opportunities when clinicians and engineers with manufacturing experience work together in close collaboration, developing designs that both suit the medical purpose and can also be made effectively.” DE