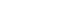U of T electrolyzer converts CO2 to ethylene at record rate
By DE Staff
Electronics SustainabilityResearchers’ enhanced device produces valuable chemicals from captured CO2 10 times faster than current technology.

This U of T-developed electrolyzer combines a copper-based catalyst with an ion-conducting polymer to convert CO2 to ethylene 10 times higher than previous designs. (Photo credit: University of Toronto / Daria Perevezentsev)
The device is similar to a fuel cell except running in reverse. In a fuel cell, hydrogen and oxygen combine on the surface of a catalyst, releasing electrons. In the electrolyzer, the electricity drives the reaction, transforming the hydrogen ions in water and CO2 into another carbon-based molecule like ethylene.
“Ethylene is one of the most widely produced chemicals in the world,” says Joshua Wicks, PhD candidate and team member along with Adnan Ozden, postdoctoral fellow F. Pelayo García de Arquer and former postdoctoral fellow Cao-Thang Dinh. “It’s used to make everything from antifreeze to lawn furniture. Today it is derived from fossil fuels, but if we could instead make it by upgrading waste CO2, it would provide a new economic incentive for capturing carbon.”
Currently, electrolyzers can’t match the production scale of fossil fuel process, due to the difficulty in combining liquid water, CO2 and electrons quickly enough and under the right conditions.
The U of T research team’ electrolyzer design speeds up the process by pairing a copper-based catalyst composed of small particles embedded in a layer of Nafion, an ion-conducing polymer commonly used in fuel cells.
“In our experiments, we discovered that a certain arrangement of Nafion can facilitate the transport of gases such as CO2,” said García de Arquer. “Our design enables gas reactants to reach the catalyst surface fast enough and in a sufficiently distributed manner to significantly increase the rate of reaction.”
While the enhanced eletrolyzer can transform CO2 into ethylene and other products 10 times faster, it does have its limitations, the team says, in that the catalyst breaks down under the devices higher-current load. According to the team, the next step will be to boost the catalyst’s durability so it lasts for thousands of hours rather than its current 10 hour lifespan. In addition, team members will also work on optimizing the system to product other carbon-based products like ethanol.
“Even if we stop using oil for energy, we are still going to need all of these molecules,” says García de Arquer. “If we can produce them using waste CO2 and renewable energy, we can have a major impact in terms of decarbonizing our economy.”
The U of T team’s work was recently published in the journal, Science.
www.engineering.utoronto.ca